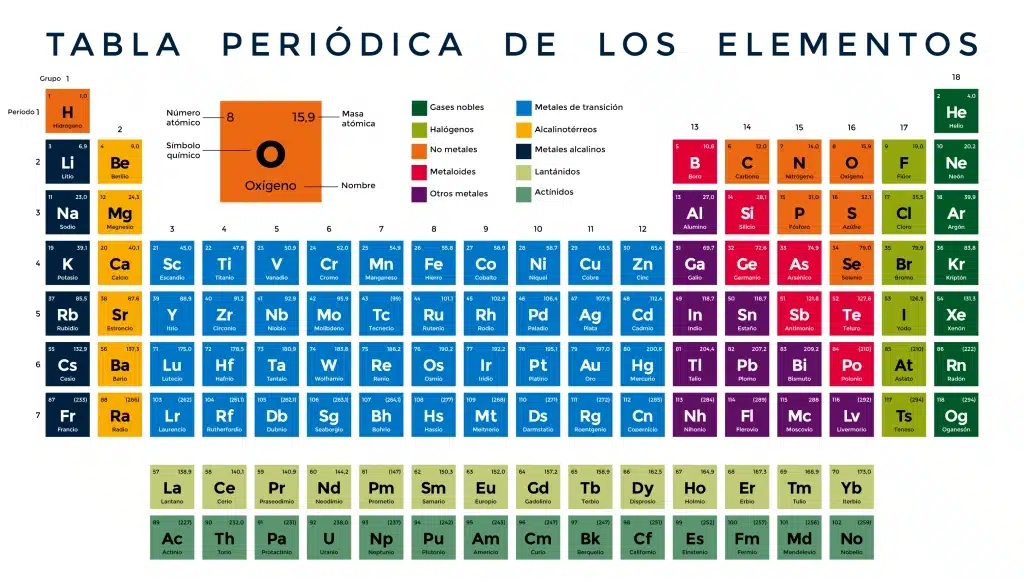
Rows of the table are called periods and columns are groups
Who published the first periodic table?
Click on the table, you'll like it. You will access a full interactive version to explore each element in detail.

Click on the table, you'll like it. You will access a full interactive version to explore each element in detail.